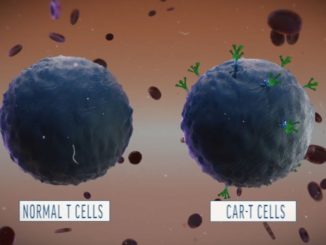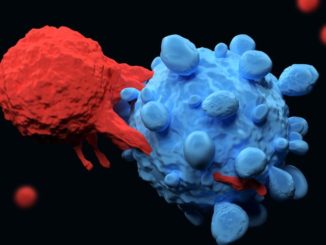
Novartis Receives Second FDA Approval for Kymriah® to Treat B-cell Lymphoma
Novartis Receives Second FDA Approval for Kymriah® to Treat B-cell Lymphoma By: Novartis News Novartis today announced the US Food and Drug Administration (FDA) has approved Kymriah® (tisagenlecleucel) suspension for intravenous infusion for its second indication – the treatment of adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two [..]