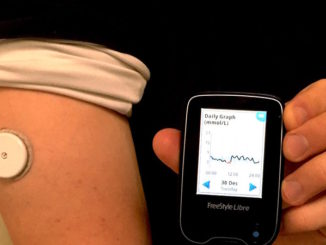
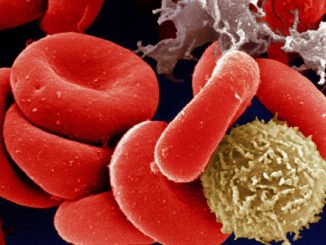
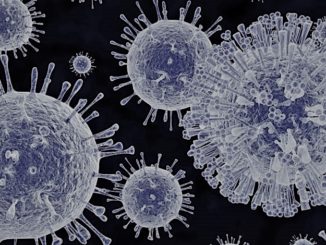
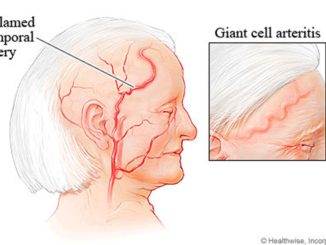
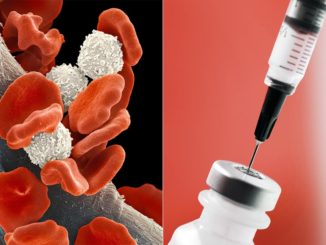
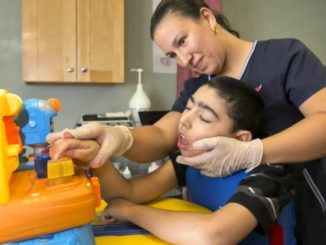
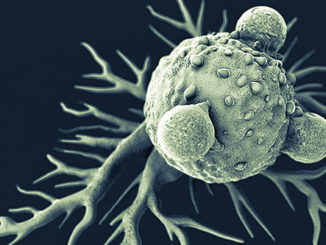
FDA Awards 15 Grants for Clinical Trials for The Product Development Against Rare Diseases
FDA Awards 15 Grants for Clinical Trials for The Product Development Against Rare Diseases By: FDA News The U.S. Food and Drug Administration announced that it has awarded 15 new clinical trial research grants totaling more than $22 million over the next four years to boost the development of products [..]