
Novel Direction Towards Diabetes Cure
By: Vijay Soni, Ph.D. | Founder and C.E.O. of Scipreneur
The research will be helpful for designing new drugs that target GIP receptors for diabetes cure
The research will be helpful for designing new drugs that target GIP receptors for diabetes cure
A team of researchers at Weill Cornell Medical College (WCMC), New York have identified a novel trafficking pathway for a G-protein-coupled receptor (GPCR, the largest family of signaling receptors). The group has studied the trafficking of the receptor (GIPR) for the hormone GIP (Glucose-dependent Insulinotropic Polypeptide). GIP is an incretin hormone, which is involved in regulation of insulin secretion together with another incretin hormone GLP-1. Agonists for these hormones are currently in clinical trials for the treatment of diabetes.
The group had previously shown that the receptor traffics in the absence of its ligand, which is uncommon for GPCRs. The receptor also has a ‘variant’ in humans that has been shown to be involved in diminished insulin secretion. Using quantitative fluorescence microscopy, the group has shown that the receptor is downregulated via sorting to the trans-Golgi network (TGN). The recycling of the receptor was found to depend on the interaction with beta arrestin-2.
Glucose-dependent insulinotropic polypeptide (GIP) is an incretin hormone involved in nutrient homeostasis.
Glucose-dependent insulinotropic polypeptide (GIP) is an incretin hormone involved in nutrient homeostasis.
According to the Dr. Timothy (the principal investigator at WCMC) “GIP receptor is an important therapeutic target for diabetes treatment. There is, however, much to be learned about how GIP binding affects the receptor behavior. Our work has revealed the underlying mechanism of GIPR trafficking and it links the E354Q-variant to altered post-activation GIP receptor trafficking. This work has implications for how we link post-activation GIP receptor trafficking to the biological function of GIP. This is a particularly important consideration when designing drugs that target GIP receptor. Non-native agonists may engage the GIP receptor in ways that induce aberrant post-activation trafficking of the receptor and thereby impact the desired biological output.”
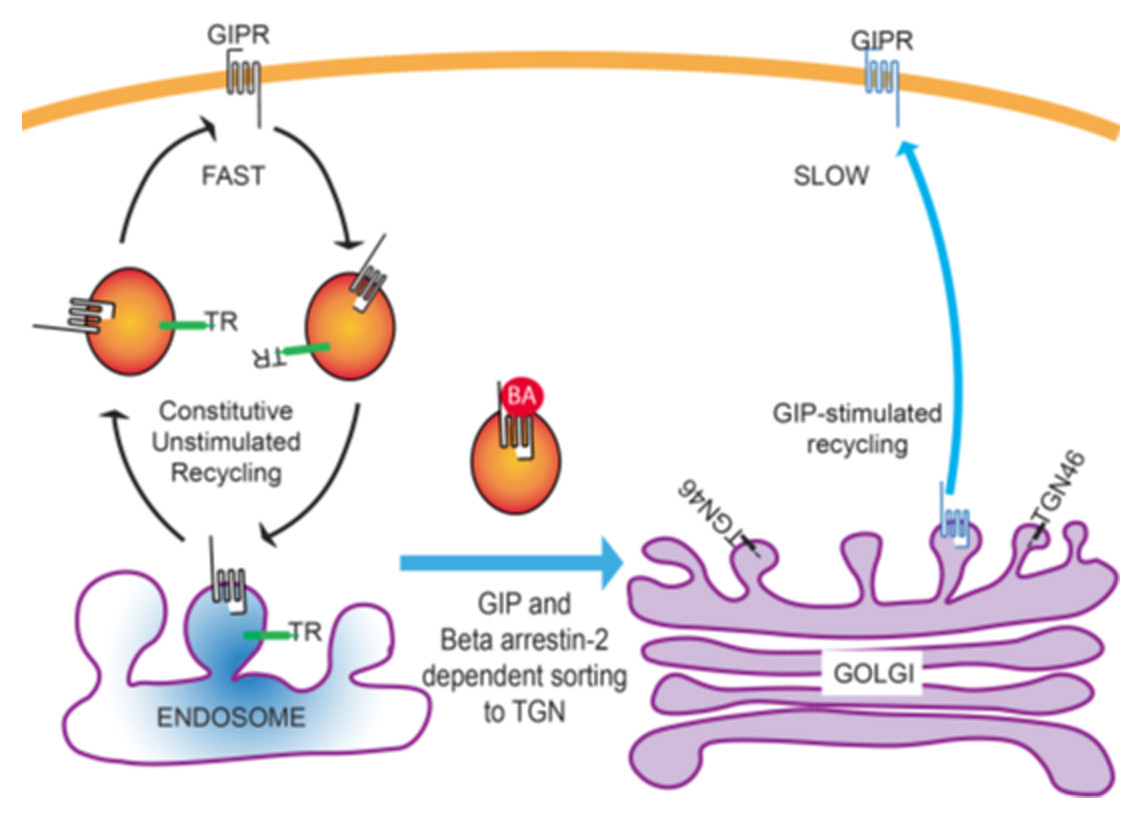
The study has been published in the journal Cell Reports. The GPCRs are the largest group of signaling receptors in mammals. Typical GPCRs are expressed at the cell membrane and undergo recycling when bound to the hormone/ligand. In contrast, the GIPR was shown to undergo recycling in the absence of the ligand. The ligand binding showed reduced exocytosis, which the group showed was dependent on binding with beta arrestin-2. The significance of this study is reflected by the fact that the mutant receptor was found to persist inside the TGN for a longer duration. This suggests that the trafficking of the receptor is important for its biological function. The molecules identified by the group for the reduced recycling of the receptor could be used as a target for therapy.
“GRKs (GPCR kinases) and beta-arrestins are known to function in ligand-induced down-regulation of many GPCRs by promoting internalization from the plasma membrane. Although GRKs and beta-arrestin2 have a role in the downregulation of GIPR, it is not by the conical mechanism of induced-internalization but rather targeting GIP activated GIPR from the rapid endosomal recycling pathway to the slow TGN recycling pathway. This altered intracellular itinerary, in addition to promoting a dynamic down-regulation from the plasma membrane, may also impact GIPR signal transduction” says Dr. Nazish Abdullah (the first author).
This work shows how versatile the GPCR trafficking is and how a mutation could affect its trafficking and overall function.
* This work was supported by NIH grant DK096925 to Prof. Timothy E. McGraw.







Leave a Reply