FDA Grants Priority Review to Genentech’s Cancer Immunotherapy Tecentriq
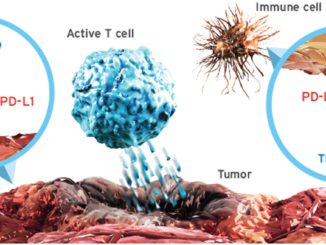
FDA Grants Priority Review to Genentech’s Cancer Immunotherapy Tecentriq By: Genentech News Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s supplemental Biologics License Application (sBLA) and granted Priority Review for TECENTRIQ® (atezolizumab), in combination [..]
