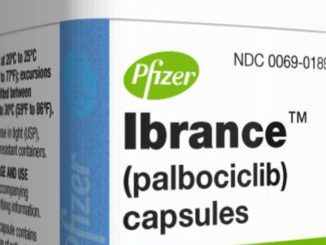
FDA Accepts Supplemental New Drug Application for Pfizer’s IBRANCE® (palbociclib) in HR+, HER2- Metastatic Breast Cancer
FDA Accepts Supplemental New Drug Application for Pfizer’s IBRANCE® (palbociclib) in HR+, HER2- Metastatic Breast Cancer December 22, 2016 Pfizer Inc. (NYSE:PFE) announced that the U.S. Food and Drug Administration (FDA) has accepted for review a supplemental New Drug Application (sNDA) for its first-in-class CDK 4/6 inhibitor, IBRANCE® (palbociclib). The [..]