New Biomarker Oncogene For The Early Detection of Breast Cancer
December 27, 2016
An oncogene discovered by NUS scientists could be used as a biomarker for the early detection and prognosis of breast cancer. This may happen as soon as next year, as the patent has been licensed to Singapore biomedical diagnostic company Restalyst Pte Ltd to be commercialised into a test kit.
The WBP2 gene, uncovered in 2011 by Assistant Professor Lim Yoon Pin of NUS Biochemistry and team, has been determined to cause the rapid proliferation of breast cancer cells. In the current study published in the journal Cancer Research last month, the Principal Investigator found that the level of the oncogene was elevated in up to 90 per cent of patients with breast cancer. Even precancerous tissue showed significantly raised level of the gene compared to normal tissues.
The WBP2 analysis is performed at the point of biopsy or surgery. The gene could be tapped as a biomarker for early detection of the cancer, said Asst Prof Lim. The test kit is based on the principle of molecular pathology, which premised that molecular transformation in cells occurs before any visible morphological changes in the cells. This may help to detect cancer earlier.
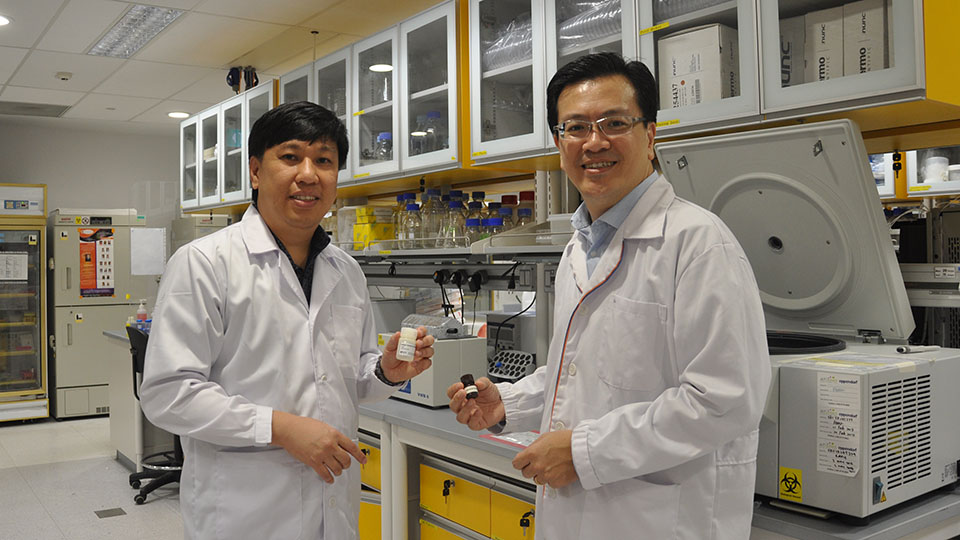
Asst Prof Lim (left) and Mr Peh holding a test kit prototype that detects the WBP2 gene causing breast cancer
The study also shows that patients with high levels of WBP2 tend to have more aggressive cancer and a higher rate of recurrence. These higher-risk patients may require closer monitoring with more customised treatment.
Another possible application would be in administering drugs or biological products that target WBP2 directly or activate regulators of the oncogene that can inhibit its expression. The researchers have identified a cancer suppressor called ITCH that stops the activity of WBP2 by destroying its protein; this could provide another approach to combat breast cancer. Such works are ongoing in Asst Prof Lim’s laboratory.
Mr Zaccheus Peh, CEO of Restalyst, pointed out that by offering a complementary test for clinicians in addition to histology and cytology, the kit could accurately detect the cancer much earlier and save more lives.
The current test kit prototype employs immunohistochemistry which does not give calibrated values of the WBP2 levels. Restalyst is developing an ELISA for WBP2 detection, an immunology screening technique that provides absolute quantification values to enhance the kit’s precision and ease-of-use for clinicians. The firm has previously created a gastric cancer screening kit using another technology developed by Dr Lim’s team.







Leave a Reply